
PDF Publication Title:
Text from PDF Page: 003
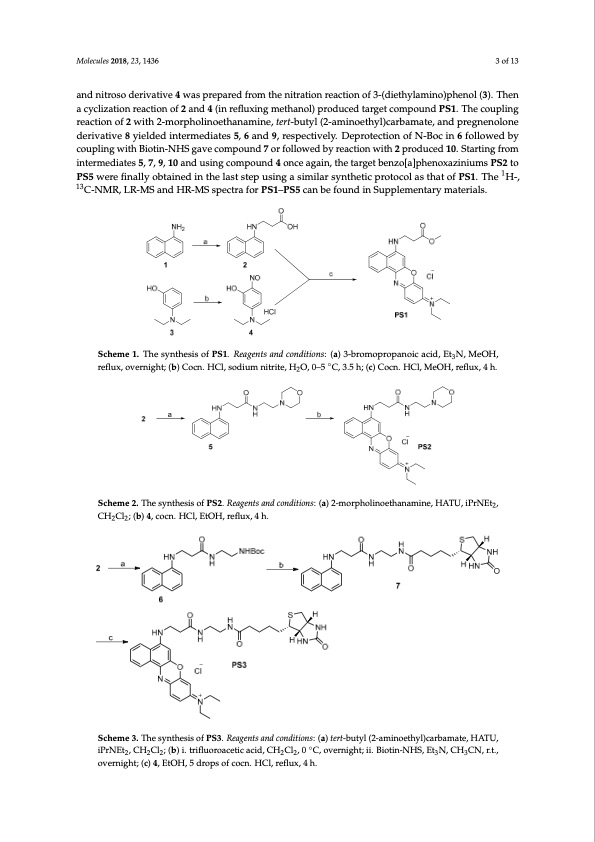
Molecules 2018, 23, x FOR PEER REVIEW 3 of 13 Molecules 2018, 23, 1436 3 of 13 acid, and nitroso derivative 4 was prepared from the nitration reaction of 3-(diethylamino)phenol (3). Molecules 2018, 23, x FOR PEER REVIEW 3 of 13 Then a cyclization reaction of 2 and 4 (in refluxing methanol) produced target compound PS1. The Molecules 2018, 23, x FOR PEER REVIEW 3 of 13 coupling reaction of 2 with 2-morpholinoethanamine, tert-butyl (2-aminoethyl)carbamate, and acid, and nitroso derivative 4 was prepared from the nitration reaction of 3-(diethylamino)phenol (3). and nitroso derivative 4 was prepared from the nitration reaction of 3-(diethylamino)phenol (3). Then acid, and nitroso derivative 4 was prepared from the nitration reaction of 3-(diethylamino)phenol (3). pregnenolone derivative 8 yielded intermediates 5, 6 and 9, respectively. Deprotection of N-Boc in 6 Then a cyclization reaction of 2 and 4 (in refluxing methanol) produced target compound PS1. The a cyclization reaction of 2 and 4 (in refluxing methanol) produced target compound PS1. The coupling Then a cyclization reaction of 2 and 4 (in refluxing methanol) produced target compound PS1. The followed by coupling with Biotin-NHS gave compound 7 or followed by reaction with 2 produced coupling reaction of 2 with 2-morpholinoethanamine, tert-butyl (2-aminoethyl)carbamate, and reaction of 2 with 2-morpholinoethanamine, tert-butyl (2-aminoethyl)carbamate, and pregnenolone coupling reaction of 2 with 2-morpholinoethanamine, tert-butyl (2-aminoethyl)carbamate, and 10. Sptarergtinegnolfornoemdeirnivteatrimvee8diyaieteldsed5,int7e,rm9e,d1ia0tesa5n,d6aunsdin9,grescpoemctpivoeulyn.dDe4protnecteionagofaiNn-,Bothcein6target derivative 8 yielded intermediates 5, 6 and 9, respectively. Deprotection of N-Boc in 6 followed by pregnenolone derivative 8 yielded intermediates 5, 6 and 9, respectively. Deprotection of N-Boc in 6 followed by coupling with Biotin-NHS gave compound 7 or followed by reaction with 2 produced benzo[a]phenoxaziniums PS2 to PS5 were finally obtained in the last step using a similar synthetic couplfionlgloweitdhbByioctoiunp-NlinHgSwgiathveBicotmin-pNoHunSdga7voercfomllopwouendb7yorreafocltliownewdibthy 2repacrtoidonucweidth102.pSrtoadruticnegdfrom 10. Starting from interm1edia1t3es 5, 7, 9, 10 and using compound 4 once again, the target protocol as that of PS1. The H-, C-NMR, LR-MS and HR-MS spectra for PS1–PS5 can be found in 10. Starting from intermediates 5, 7, 9, 10 and using compound 4 once again, the target intermediates 5, 7, 9, 10 and using compound 4 once again, the target benzo[a]phenoxaziniums PS2 to benzo[a]phenoxaziniums PS2 to PS5 were finally obtained in the last step using a similar synthetic Supplementary materials. benzo[a]phenoxaziniums PS2 to PS5 were finally obtained in the last step using a similar synthetic 1 PS5werefinallyobtainedinth1ela1s3tstepusingasimilarsyntheticprotocolasthatofPS1.The H-, 13 protocol as that of PS1. The H-, C-NMR, LR-MS and HR-MS spectra for PS1–PS5 can be found in protocol as that of PS1. The 1H-, 13C-NMR, LR-MS and HR-MS spectra for PS1–PS5 can be found in C-NSMupRp,leLmRe-nMtaSryanmdatHerRia-lMs.SspectraforPS1–PS5canbefoundinSupplementarymaterials. Supplementary materials. Scheme 1. The synthesis of PS1. Reagents and conditions: (a) 3-bromopropanoic acid, Et3N, MeOH, Scheme 1. The synthesis of PS1. Reagents and conditions: (a) 3-bromopropanoic acid, Et3N, MeOH, Scheme 1. The synthesis of PS1. Reagents and conditions: (a) 3-bromopropanoic acid, Et3N, MeOH, Scheme 1. The synthesis of PS1. Reagents and conditions: (a) 3-bromopropanoic acid, Et3N, MeOH, reflux, overnight; (b) Cocn. HCl, sodium nitrite, H2O, 0–5 °C, 3.5 h; (c) Cocn. HCl, MeOH, reflux, 4 h. reflux, overnight; (b) Cocn. HCl, sodium nitrite, H2O, 0–5 °C, 3.5 h; (c) Cocn. HCl, MeOH, reflux, 4 h. reflux, overnight; (b) Cocn. HCl, sodium nitrite, H O, 0–5 ◦C, 3.5 h; (c) Cocn. HCl, MeOH, reflux, 4 h. reflux, overnight; (b) Cocn. HCl, sodium nitrite, H2O, 0–5 °C, 3.5 h; (c) Cocn. HCl, MeOH, reflux, 4 h. Scheme 2. The synthesis of PS2. Reagents and conditions: (a) 2-morpholinoethanamine, HATU, iPrNEt2, Scheme 2. The synthesis of PS2. Reagents and conditions: (a) 2-morpholinoethanamine, HATU, iPrNEt2, CH2Cl2; (b) 4, cocn. HCl, EtOH, reflux, 4 h. Scheme 2. ThesynthesisofPS2..Reeaageenttsaandccoondiittiions::((aa))22--morrpholliinoethanamine,HATU, iPrNEt2, CH2Cl2; (b) 4, cocn. HCl, EtOH, reflux, 4 h. CH2Cl2;; ((b)) 4,, cocn.. HCll,,EttOH,,rreffllux,, 4 h.. overnight; (c) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h. Scheme 3. The synthesis of PS3. Reagents and conditions: (a) tert-butyl (2-aminoethyl)carbamate, HATU, Scheme 3. The synthesis of PS3. Reagents and conditions: (a) tert-butyl (2-aminoethyl)carbamate, HATU, iPrNEt2, CH2Cl2; (b) i. trifluoroacetic acid, CH2Cl2, 0 °◦C, overnight; ii. Biotin-NHS, Et3N, CH3CN, r.t., iPrNEt2, CH2Cl2; (b) i. trifluoroacetic acid, CH2Cl2, 0 C, overnight; ii. Biotin-NHS, Et3N, CH3CN, r.t., overnight; (c) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h. overnight; (c) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h. 22 2 Scheme 3. The synthesis of PS3. Reagents and conditions: (a) tert-butyl (2-aminoethyl)carbamate, HATU, Scheme 3. The synthesis of PS3. Reagents and conditions: (a) tert-butyl (2-aminoethyl)carbamate, HATU, iPrNEt2, CH2Cl2; (b) i. trifluoroacetic acid, CH2Cl2, 0 °C, overnight; ii. Biotin-NHS, Et3N, CH3CN, r.t., iPrNEt2, CH2Cl2; (b) i. trifluoroacetic acid, CH2Cl2, 0 °C, overnight; ii. Biotin-NHS, Et3N, CH3CN, r.t., overnight; (c) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h.PDF Image | Photosensitizers for Anticancer Photodynamic Therapy
PDF Search Title:
Photosensitizers for Anticancer Photodynamic TherapyOriginal File Name Searched:
molecules-23-01436-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Cruise Ship Reviews | Luxury Resort | Jet | Yacht | and Travel Tech More Info
Cruising Review Topics and Articles More Info
Software based on Filemaker for the travel industry More Info
The Burgenstock Resort: Reviews on CruisingReview website... More Info
Resort Reviews: World Class resorts... More Info
The Riffelalp Resort: Reviews on CruisingReview website... More Info
| CONTACT TEL: 608-238-6001 Email: greg@cruisingreview.com | RSS | AMP |