
PDF Publication Title:
Text from PDF Page: 004
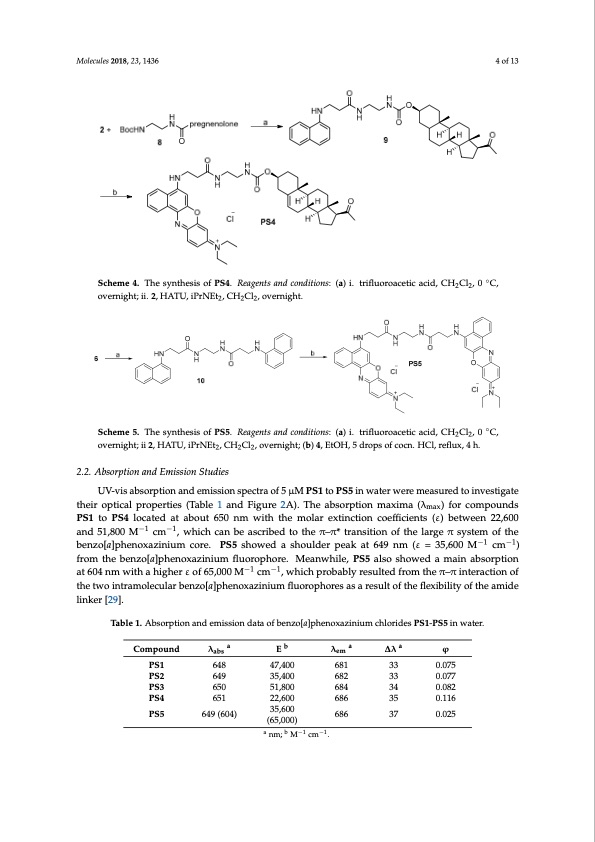
Molecules 2018, 23, 1436 Molecules 2018, 23, x FOR PEER REVIEW 4 of 13 Molecules 2018, 23, x FOR PEER REVIEW 4 of 13 4 of 13 Scheme 4. The synthesis of PS4. Reagents and conditions: (a) i. trifluoroacetic acid, CH2Cl2, 0 ◦°C, Scheme 4. The synthesis of PS4. Reagents and conditions: (a) i. trifluoroacetic acid, CH Cl , 0 C, Scheme 4. The synthesis of PS4. Reagents and conditions: (a) i. trifluoroacetic acid, CH2Cl2, 0 °C, overnight; ii. 2, HATU, iPrNEt2, CH2Cl2, overnight. overnight; ii. 2, HATU, iPrNEt , CH Cl , overnight. overnight; ii. 2, HATU, iPrNEt2, CH2Cl22, overnight. Scheme 5. The synthesis of PS5. Reagents and conditions: (a) i. trifluoroacetic acid, CH2Cl2, 0 °C, Scheme 5. The synthesis of PS5. Reagents and conditions: (a) i. trifluoroacetic acid, CH2Cl2, 0 °◦C, Scheme 5. The synthesis of PS5. Reagents and conditions: (a) i. trifluoroacetic acid, CH2Cl2, 0 C, overnight; ii 2, HATU, iPrNEt2, CH2Cl2, overnight; (b) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h. overnight; ii 2, HATU, iPrNEt2, CH2Cl2, overnight; (b) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h. overnight; ii 2, HATU, iPrNEt2, CH2Cl2, overnight; (b) 4, EtOH, 5 drops of cocn. HCl, reflux, 4 h. 2.2. Absorption and Emission Studies 2.2. Absorption and Emission Studies 2.2. Absorption and Emission Studies UV-vis absorption and emission spectra of 5 μM PS1 to PS5 in water were measured to UV-vis absorption and emission spectra of 5 μM PS1 to PS5 in water were measured to UV-vis absorption and emission spectra of 5 μM PS1 to PS5 in water were measured to investigate investigate their optical properties (Table 1 and Figure 2A). The absorption maxima (λmax) for investigate their optical properties (Table 1 and Figure 2A). The absorption maxima (λmax) for their optical properties (Table 1 and Figure 2A). The absorption maxima (λ ) for compounds compounds PS1 to PS4 located at about 650 nm with the molar extinction coemffaixcients (ε) between compounds PS1 to PS4 located at about 650 nm with the molar extinction coefficients (ε) between PS1 to PS4 located−1at a−b1out 650 nm with the molar extinction coefficients (ε) between 22,600 22,600 and 51,800 M cm , which can be ascribed to the π–π* transition of the large π system of the 22,600 and 51,800 M−1 cm−1, which can be ascribed to the π–π* transition of the large π system of the and 51,800 M−1 cm−1, which can be ascribed to the π–π* transition of the large π−1 sys−t1em of the benzo[a]phenoxazinium core. PS5 showed a shoulder peak at 649 nm (ε = 35,600 M cm ) from the benzo[a]phenoxazinium core. PS5 showed a shoulder peak at 649 nm (ε = 35,600 M−1 cm−1) from the benzo[a]phenoxazinium core. PS5 showed a shoulder peak at 649 nm (ε = 35,600 M−1 cm−1) benzo[a]phenoxazinium fluorophore. Meanwhile, PS5 also showed a main absorption at 604 nm with benzo[a]phenoxazinium fluorophore. Meanwhile, PS5 also showed a main absorption at 604 nm with fromthebenzo[a]phenox−1azini−u1mfluorophore.Meanwhile,PS5alsoshowedamainabsorption a higher ε of 65,000 M cm , which probably resulted from the π–π interaction of the two a higher ε of 65,000 M−1 cm−1, which probably resulted from the π–π interaction of the two at 604 nm with a higher ε of 65,000 M−1 cm−1, which probably resulted from the π–π interaction of intramolecular benzo[a]phenoxazinium fluorophores as a result of the flexibility of the amide linker intramolecular benzo[a]phenoxazinium fluorophores as a result of the flexibility of the amide linker the two intramolecular benzo[a]phenoxazinium fluorophores as a result of the flexibility of the amide [29]. [29]. linker [29]. Under excitation at 600 nm, all these compounds exhibited near-infrared emissions at about 681 Under excitation at 600 nm, all these compounds exhibited near-infrared emissions at about 681 nm, with Stokes shifts of about 35 nm (Figure 2B). In addition, the relative fluorescence quantum nm, with Stokes shifts of about 35 nm (Figure 2B). In addition, the relative fluorescence quantum Table 1. Absorption and emission data of benzo[a]phenoxazinium chlorides PS1-PS5 in water. yields (φ) were measured in water using fluorescein as a standard (φs = 0.98) [30]. These compounds yields (φ) were measured in water using fluorescein as a standard (φs = 0.98) [30]. These compounds all showed relatively low fluorescence quantum yields (0.025 to 0.116), which is consistent with the abaa all showed reClaotmivpeoluynldow fluoλrescence quantuEm yields (0.λ025 to 0.116∆),λwhich is coφnsistent with the abs em result of previously reported benzo[a]phenoxazinium chlorides [13]. This result indicates that PS1– result of previously reported benzo[a]phenoxazinium chlorides [13]. This result indicates that PS1– PS1 648 47,400 PS5 may not tend to decay back to the ground state by emitting fluorescence after excitation and have PS5 may not tendPSto2 decay back6t4o9 the groun3d5s,4t0a0te by emitt6i8n2g fluoresce3n3 ce after e0x.0c7it7ation and have a great potential in undergoing intersystem crossing to form a relatively long-lived triplet state, which PS3 650 51,800 a great potential in undergoing intersystem crossing to form a relatively long-lived triplet state, which 0.116 0.025 is necessary for acting as desirable PDT candidates. 684 34 0.082 PS4 651 22,600 686 35 is necessary for acting as desirable PDT candidates. PS5 649 (604) 35,600 686 37 (65,000) a nm; b M−1 cm−1. 681 33 0.075PDF Image | Photosensitizers for Anticancer Photodynamic Therapy
PDF Search Title:
Photosensitizers for Anticancer Photodynamic TherapyOriginal File Name Searched:
molecules-23-01436-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Cruise Ship Reviews | Luxury Resort | Jet | Yacht | and Travel Tech More Info
Cruising Review Topics and Articles More Info
Software based on Filemaker for the travel industry More Info
The Burgenstock Resort: Reviews on CruisingReview website... More Info
Resort Reviews: World Class resorts... More Info
The Riffelalp Resort: Reviews on CruisingReview website... More Info
| CONTACT TEL: 608-238-6001 Email: greg@cruisingreview.com | RSS | AMP |